Signed in as:
filler@godaddy.com
Signed in as:
filler@godaddy.com

Lightpoint today announces a world-first bladder cancer sentinel lymph node (SLN) procedure with SENSEI®, Telix's miniature robotic-assisted gamma probe used to detect radiation in patients and guide surgery. The patient was operated on at Hospital del Mar in Barcelona, Spain.
.jpg/:/cr=t:0%25,l:0%25,w:100%25,h:100%25/rs=w:388,cg:true)
Congratulations to Professor Ronald Zweemer & Dr Kees Gerestein for publication of their clinical study results using SENSEI® in robot-assisted sentinel lymph node detection.
Congratulations to the team at UCLA Health in California for first use of SENSEI® in robot-assisted prostate cancer surgery.

SENSEI® recognized as the Best New Surgical Technology Solution in the 2022 MedTech Breakthrough Awards.

Professor Manish Chand launches clinical feasibility study using SENSEI® in robot-assisted colorectal cancer surgery at University College Hospital, London.

Dr Doug Adams, Bethesda North Hospital, Cincinatti, Ohio (part of TriHealth) performs the first procedures with SENSEI® in lung surgery and first use in the US.

Lightpoint announces positive clinical trial data for SENSEI® in prostate cancer surgery at the Annual Congress of the European Association of Nuclear Medicine. SENSEI® achieved 100% detection of sentinel lymph nodes in prostate cancer surgery and detected more sentinel nodes than a conventional gamma probe.

Dr Matthias Heck at the Technical University of Munich is the world's first surgeon to test SENSEI® in combination with PSMA in prostate cancer surgery.

Lightpoint signs commercial agreement with HOAM Medical (www.hoammedi.com) for distribution of SENSEI®, our miniature gamma probe for minimally-invasive and robot-assisted surgery, in South Korea.
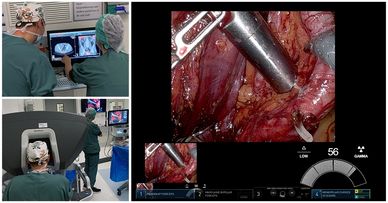
The brilliant team at UZ Leuven completed their first patient to help us evaluate SENSEI®, our miniature gamma probe for minimally-invasive and robot assisted surgery, in the treatment of prostate cancer.

Lightpoint and Telix will work together to explore how Lightpoint's SENSEI® surgical probe and Telix's molecular imaging agents may be used together for real-time, intra-operative cancer detection.

Dr Jochen Walz and his team at the Institut Paoli-Calmettes in Marseille are the first team in France to use SENSEI® with the da Vinci surgical robot.

Surgeon's Choice will be responsible for the distribution and sale of SENSEI® across Australia.

TECNASA, Technologias Asociadas will be responsible for the distribution and sale of SENSEI® in Spain and Portugal.

SENSEI®, our miniaturized probe for minimally-invasive and robot-assisted cancer surgery, has been successfully registered with the FDA and is now authorized for sale in the US.

SENSEI®, Lightpoint's miniaturized probe designed for intra-operative detection of sentinel lymph nodes as well as cancer metastasis through the lymphatic system, has received regulatory approval in the EU.